
Here are three classic examples of nucleophilic acyl substitution reactions that work well. Three Nucleophilic Acyl Substitution Reactions (That Work Pretty Well) In a subsequent article we’ll cover cases where an acid catalyst is employed for nucleophilic acyl substitution, such as the Fischer esterification, acidic hydrolysis of esters, and many other examples. In this article we will specifically cover examples of nucleophilic acyl substitution reactions with negatively charged nucleophiles. Hover to see other families of nucleophilic substitution reactions A carbon-nucleophile bond forms, and a carbon-leaving group bond breaks.Īlthough the mechanism is different, nucleophilic acyl substitution superficially resembles substitution reactions we’ve seen before such as nucleophilic aliphatic substitutionand nucleophilic aromatic substitution. This is classified as a substitution reaction because we are forming and breaking a bond on the same carbon. Nucleophilic acyl substitution is a reaction where a nucleophile forms a new bond with the carbonyl carbon of an acyl group with accompanying breakage of a bond between the carbonyl carbon and a leaving group. (Advanced) References and Further Reading.Neutral Nucleophiles and Acid Catalysis.Grignard Reagents and LiAlH 4 Add Twice.Intramolecular Nucleophilic Acyl Substitution.NAS Reactions Are Favored When The Leaving Group Is A Weaker Base Than The Nucleophile.Some Nucleophilic Acyl Substitutions That DON’T Work.The Addition-Elimination Mechanism for Nucleophilic Acyl Substitution With a Negatively Charged Nucleophile.Three Nucleophilic Acyl Substitution Reactions (That Work Pretty Well).
#Carboxylic acid derivatives reactivity plus
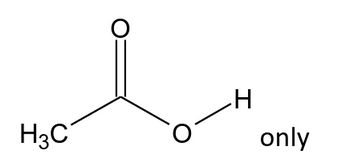
If you recall that “stronger acid plus stronger base gives weaker acid plus weaker base”, you’ll be well on your way to being able to predict whether or not a given nucleophilic acyl substitution reaction will happen. furthermore, for all intents and purposes, NAS reactions behave a lot like acid-base reactions.with negatively charged nucleophiles nucleophilic acyl substitution tends to follow a simple two-step mechanism (addition-elimination).Yes, there are a lot of reactions of carboxylic acid derivatives to learn! In this article we’ll explore what is hands-down the most important pathway: nucleophilic acyl substitution. Nucleophilic Acyl Substitution (With Negatively Charged Nucleophiles)


 0 kommentar(er)
0 kommentar(er)
